Executive Summary
A major theme in early childhood education is that brain research has established the importance of early windows of opportunity that can be exploited to assure optimal brain development and life-long well-being. Explanations involving brain science have a seductive appeal, especially among the general public and policy-makers. Thus, neuroscientific evidence requires special scrutiny in the policy realm. Consideration of the neuroscience behind claims about windows of opportunity reveals a contrast between what is claimed in the policy as opposed to the scholarly literature. The advocacy literature tends to tell only half of the story about the effects of experience on synapse formation. The full story raises doubts as to how much specific guidance neuroscience can provide policy makers about what should go into those windows of opportunity.
Introduction
President Obama’s “Preschool for All” initiative, announced in his 2013 State of the Union address, proposed $75 billion in new federal funding over ten years to assure access to high quality early childhood education. Brain science, he said, provides support for this initiative: “Research has shown that the early years in a child’s life—when the human brain is forming—represent a critically important window of opportunity to develop a child’s full potential and shape key academic, social, and cognitive skills that determine a child’s success in school and in life.”[i]
On October 6, 2015 the President and CEO of the New York Academy of Sciences wrote, “New research tells us how to take advantage of the earliest window of opportunity for childhood development – the first two years. Now we need to act on it.”[ii]
Since ideas from neuroscience were introduced into the early childhood policy arena in the mid-1990s, we have become so accustomed to hearing about early brain development and “windows of opportunity” that we rarely pause to consider the neuroscientific evidence behind these assertions.
We should always be open to considering the evidence – that is the purpose of Evidence Speaks – but evaluating neuroscientific evidence in policy formation is particularly important. Psychological research has found that explanations of human behavior generate more public interest when neuroscience is incorporated into those explanations. Explanations that include neuroscience have a seductive appeal. The inclusion of brain science “may encourage people to believe they have received a scientific explanation when they have not.” [iii] Neuroscience has a particularly strong effect on non-experts’ judgments, causing them to accept explanations they might otherwise reject. So, there is additional incentive to assess explanations and arguments that incorporate neuroscientific information. If the psychologists are correct, when neuroscience is introduced into policy debates, it may influence opinion beyond what the evidence can support.
Reviewing all the neuroscience that has been introduced into the realm of early childhood education policy would be a massive undertaking. Here I want to examine how just one neuroscientific finding is being used within early childhood policy circles. The finding is this: At birth, there is a rapid increase in the number of neural connections, or synapses, in the developing brain (developmental synaptogenesis), followed by a protracted period of synapse elimination lasting until at least adolescence in some brain areas. This finding comes from basic neuroscientific research at the level of nerve cells, cell structures, and molecules. This work is at a much more basic level of analysis than, for example, that of cognitive neuroscience and brain imaging. However, it is a finding fundamental to claims about the importance and policy significance of early windows of opportunity.
To examine how this basic neuroscience is used as evidence in policy discussions, I will focus on the figures and graphs used to illustrate this neuroscientific phenomenon. What the graphs and figures include or exclude in their portrayals of major events in brain development and what the accompanying texts say about the figures are revealing. We will find that scholarly presentations and discussions of developmental synaptogenesis differ from those found in the policy literature; that what is known about developmental synaptogenesis and pruning provides little specific guidance to parents, educators, and policy makers; and that the policy literature tends to overemphasize one type of synaptic change at the cost of ignoring another equally important type. Maybe this is a case where neuroscience is influencing opinion beyond what the evidence can support.
The Blooming and Pruning of Neural Connections
Ideas about synapse growth and elimination in brain development are so central to the early childhood policy literature that figures showing the course of synaptic blooming and pruning have become iconic within the early childhood policy literature. The proto-icon is Figure 1 prepared by Charles A. Nelson, now at Harvard Medical School, for the National Research Council – Institute of Medicine document From Neurons to Neighborhoods (hereafter, N2N).[iv]
Nelson’s figure, entitled of “Human Brain Development,” portrays three significant findings from developmental neuroscience. Starting at the top, the figure shows the presence of experience-dependent synapse formation throughout the life span. As we will see, this type of synapse formation is not discussed in the early childhood literature to the same extent as changing synaptic densities, even though, as the figure shows, this kind of synapse formation begins at birth. Next, the figure refers to neurogenesis in the hippocampus. This is a relatively recent finding. Previously neuroscientists thought that all the neurons a person would ever have were present at birth. In recent years, they have established that new neurons form later in life within the hippocampus, a brain area associate with memory formation. This is an important finding, but one that does not figure prominently in the early childhood literature.
The most striking feature of the figure is the three curves representing the phenomenon of synaptic blooming and pruning in three brain areas– sensory cortex, angular gyrus – Broca’s area, and prefrontal cortex. These three brain areas are associated with sensory systems (vision, hearing), with perceiving and producing speech, and with higher cognitive functions. Higher cognitive functions are cognitive skills that require keeping information in mind for a period of time – working memory, mental imagery, decision making, choosing an action.
What does N2N say about Figure 1? Since the 1970s, based on research in cats and monkeys, neuroscientists have known about the phenomenon of synaptic blooming and pruning. Figure 1, however, shows the human data. Peter Huttenlocher and his colleagues at the University of Chicago, spent decades acquiring these data.[v] Using brain tissue available from autopsies, the scientists counted the number of synapses per unit volume of brain tissue. Synaptic densities can change over time, because synapses are added or disappear, or because other types of cells also appear during development. As N2N notes, interpreting the significance of changes in synaptic densities is exceedingly difficult. Furthermore, N2N states current evidence is not sufficient to determine how widespread synaptic blooming and pruning are in brain development generally, or in human brain development specifically.
Exactly what synaptic blooming and pruning mean for brain function and behavior is still under investigation. N2N says that most of the information we have about this structure-function relationship comes from work on animal visual systems. For example, David Hubel and Torsten Wiesel showed that normal development in one area of the cat visual system required balanced visual input to both eyes during a specific critical, or sensitive, period.[vi] They also found that normal development involves elimination of some synapses. It seems then that the type of neural tuning involving synapse elimination occurs during the period of increased synaptic densities. N2N proceeds to mention that in sensory systems, at least, the effects of experience become increasingly irreversible as animals age, synaptic densities fall, and the sensitive periods close. Hence, Huttenlocher’s curves are taken to represent times in development during which sensitive periods occur. During sensitive periods, for sensory systems at least, normal experiences result in normal development, abnormal experience in abnormal development. After the sensitive periods close, the same experiences have no or little effect on the neural connections and those connections become permanent. Sensitive periods, so the argument goes, provide windows of opportunity during which the brain is highly malleable and particularly sensitive to experience.
Of course the fact the synaptogenesis occurs and sensitive periods exist, does not tell us why evolution settled on these mechanisms. To address this question N2N, as do most other discussions of synaptogenesis in brain development, appeals to a distinction made by William Greenough and his colleagues.[vii] As N2N explains, Greenough posited the existence of two types of synaptic change, or plasticity, in the brain. The first type Greenough called experience-expectant synapse change: the over-production of synapses early in life, followed by their selective elimination, allows an organism to adjust or tune its neural circuits to its species-specific environment. Neural systems have evolved to expect certain types of stimuli in the environment, such as patterned visual stimuli, that re-enforce some neural connections and eliminate others. The three curves in Figure 1 show periods during development when experience-expectant synaptic change is likely to occur. It is generally accepted that the experiences needed to affect this neural fine-tuning are ubiquitous in all normal human environments. As N2N states, “all brains depend on the same basic experiences to develop normally” (190).
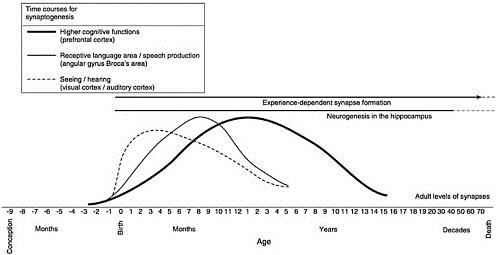
The second type of synaptic change Greenough called experience-dependent synapse formation, the type of change Nelson showed at the top of Figure 1. Experience-dependent change occurs throughout the lifespan. Unlike experience-expectant change, which involves pruning synapses, experience-dependent change involves growth of new synapses. Experience-dependent change allows individuals to encode and learn from information that occurs in their own personal, social, and cultural environments. It is the mechanism that enables us to learn throughout life.
In its chapter on the developing brain, N2N provides a reasonably measured presentation of the neuroscientific research that might be relevant to framing improved early childhood and education policies. It cautions that most of what we know about brain development is based on animal studies and that these studies may not be immediately translatable to humans. It acknowledges that interpreting data on synaptic densities in humans is difficult or even inappropriate when considering development of cognition, language, emotion and social behavior. The chapter states that a few, not all, aspects of brain development require particular experiences at particular times; that sensitive or critical periods in human development are largely unexplored; that the experiences needed for normal brain development are ubiquitous; and that the brain remains open to modification by experience throughout the lifespan.
“As a result, assertions that the die has been cast by the time the child enters school are not supported by neuroscience evidence and can create unwarranted pessimism about the potential efficacy of interventions that are initiated after the preschool years” (218). N2N acknowledges that the “challenge of deciphering what this information means for what parents, guardians, and teachers of young children should do is enormous” (183). Nonetheless N2N expresses a view that early experiences are particularly important in brain development and that they determine the course of future development and learning.
Blooming and Pruning in the Advocacy Literature
Slightly different versions of Nelson’s figure, most of which cite Nelson as the source, are more commonly found in the policy-advocacy literature and in science news stories.[viii] An Internet search yields dozens of variations on the basic theme. The version shown in Figure 2 is taken from the website of Harvard University’s Center on the Developing Child.[ix] Most of these adaptations differ only slightly from Nelson’s original. Ongoing neurogenesis in the hippocampus does not appear in the figure. More interestingly, neither does the occurrence of life-long experience-dependent synapse formation. The texts explaining Figure 2 also differ from that found in N2N. I will focus on the discussions of Figure 2 emanating from the Harvard Center, because these are the most influential and widely cited.
The working paper in which Figure 2 appears is Working Paper 5, “The Timing and Quality of Early Experiences Combine to Shape Brain Architecture”.[ix] Nelson is again credited as the source of the figure. In the working paper, the figure’s title is “Synapse Formation in the Developing Brain.” Although the figure appears in the paper, there is no explicit reference to it in the text, nor is there any detailed discussion or explanation of it. The working paper does not mention the source for data (Huttenlocher’s work) nor the difficulties inherent (according to N2N) in interpreting these data. However, developmental synaptogenesis is cited as one of three factors that explain why the developing brain is exceptionally flexible: “the brain develops far more extensive connections than it needs in order to function optimally, and connections that are not useful are pruned away over time” (2).
The other two factors, also related to synaptic blooming and pruning, are (i) the highly active molecular environment and cellular mechanisms present during synapse formation and elimination, and (ii) the finding that neural circuits are more flexible before a particular pattern of connections has been shaped and fully activated. All of the scientific work cited in support of these three explanatory factors is research on sensory systems in animals. However, the working paper is explicit in making the connection between flexible circuits and policy: “Because specific experiences affect specific brain circuits during specific developmental stages – referred to as sensitive periods – it is vitally important to take advantage of the early opportunities in the developmental building process’ (1). Hence, President Obama’s “windows of opportunity.”
What differentiates the working paper from N2N is the lack of any qualifications about the generalizability of the neuroscience it cites. There are no cautions about the advisability of generalizing from animal studies to humans, no acknowledgement that most of the work cited is on sensory systems, and no mention of experience-dependent brain plasticity over the life span. For example, caution about generalizing from animals to humans in areas of social, emotional and cognitive development give way to claims that there are sensitive periods for social cues, where these claims are supported by citing research on imprinting in chicks and rearing of rat pups. Yet despite all the brain science, in its discussion of the science-policy gap, the working paper states: “We don’t need sophisticated research to prove that aggressive preschoolers are easier to ‘rehabilitate’ than violent criminals. Common sense tells us that the learning and behavior problems of young children can be fixed more easily and at less cost than those of adolescents and young adults. Neuroscience tells us why the statements are all true” (6).
A More Considered Discussion of Nelson’s Figure
Shortly after Figure 1 appeared in N2N, Nelson himself, along with Ross Thompson from the University of Nebraska, published an article containing their version of Figure 1.[x] The article’s objective was to address misunderstandings of developmental neuroscience relevant to early childhood that were prevalent in the media circa 2001. Thompson and Nelson present a critical review of the neuroscience and offer advice to scientists and professional organizations on how to assure accurate and timely coverage of scientific findings in the media. They were particularly concerned with the perils of what they called “campaign coverage,” cases where a public relations campaign supplies information to the media in support of a particular viewpoint. As the authors say, “campaign journalism begins not with the findings of relevant research but rather with the goals of an advocacy effort” (6).
In their paper, they describe Figure 1 as portraying major events in brain development. Their version contains all the items shown in Figure 1, but it also depicts important prenatal events, such as neural tube formation and cell migration, as well as myelination that occur from two months prenatally until 10 years of age.
Thompson and Nelson present their critical review of developmental synaptogenesis in an early section of their paper. They reprise the standard presentation of synaptic overproduction and pruning, citing the human work of Huttenlocher and the monkey work of Rakic.[xi] They present Greenough’s interpretation of this phenomenon as experience-expectant plasticity and his rodent research as providing supporting evidence. However, they caution that while this process no doubt occurs in human development, it is not clear how extensively it occurs, in which brain regions it occurs, and at which times in development it occurs.
As they point out, part of the problem is that Huttenlocher’s data was derived from available human autopsy specimens. There were relatively few specimens available for some age ranges and not every brain region was studied. Furthermore, they caution, synaptic density counts do not tell us whether the synapses counted are due to a genetic program or to experience. Thompson and Nelson grant that the blooming and pruning phenomenon generalizes to human brain development, at least to the sensory systems. However, human data is not clear about the relative influence of genetic guidance versus experiential exposure for changes in synaptic density in most brain regions.
The biggest difference between Thompson and Nelson’s treatment of the brain science and the treatments found in N2N and the advocacy literature is indicated by the heading under which they present the neuroscience: “The Early Experiences Essential to Brain Development are Largely Unknown.” As we saw above, experience-expectant brain changes rely on stimuli that are ubiquitous in normal human environments. And, no doubt, different experiences contribute to the development of different brain regions. However, Thompson and Nelson observe “it is unknown what most of those experiences are” (9).
We know very little about what types of experiences are most influential in brain development and even less about the timing of those experiences. Doing adequately controlled studies about the timing of experiences is difficult in animals and even more so in humans for both methodological and ethical reasons. Thus, they conclude, it is difficult to identify experiences of critical importance in brain development and to specify precisely when these experiences must occur. In their view, based on neuroscientific evidence, one can neither provide parents and policy-makers with definitive guidance about which parental and educational practices are most beneficial to the developing brain, nor specify when particular experiences should be provided.
The inability to be specific about experiences and their timing carries over into concerns about critical periods, according to Thompson and Nelson. Critical periods are exceptional not typical in human brain development. Speech perception and exposure to patterned visual information are essential for normal brain development, but it is not clear the extent to which sensory systems can serve as model systems for other aspects of brain development. Our knowledge from developmental neuroscience about cognitive and socio-emotional development is extremely limited. In the area of cognition, relatively little is known about memory development, a cognitive ability fundamental to learning. As to windows of opportunity: “Windows of opportunity for early stimulation better characterize basic sensory and motor capacities than higher mental and personality processes, and even so, most such windows close very slowly with development. … [I]t is unclear what specific experiences are important, and when they must occur, for healthy brain development” (12). There may be windows of opportunity, but neuroscience cannot presently tell us what to put in them.
If this is the case, then neuroscience can tell us little, if anything, about specific policies – about the merits of universal pre-K versus targeted interventions, of two years of pre-K versus one, of home-based versus center-based programs. It can tell us little about curricula or teacher training.
Moving away from experience-expectant brain plasticity, they point to the under-reported, under-emphasized implications of experience-dependent synapse formation: Brain development is life-long. If so, even if we could specify the kinds and timing of experiences contributing to optimal early brain development, this does not ensure the course of development in future years. They worry that over-emphasizing early development minimizes the importance and extent of later brain development, later brain changes, and later experience. Focusing on experience-expectant change alone could lead one to mistakenly attribute later brain changes to life-long effects of early formative influences, rather than to the later experiences themselves. In their view, another implication of life-long, experience-dependent brain change is that with appropriate interventions, early deprivation and harm might be remediable in later years.
Academic versus Advocate
Thompson and Nelson’s critical review of the neuroscience is more guarded than that appearing in N2N, but it is in the same ballpark. In comparison, the policy and advocacy literature is at best in a neighboring parking lot. In that literature, we lose any sense of the problems in generalizing from animal models to humans, the limitations of using sensory systems as general models of brain development, and even of the inherent difficulties of interpreting the limited data we have on changes in human synaptic densities over the life span. However, the biggest difference is evident in the figures they use to illustrate their presentations. The policy-advocacy literature tends to omit the mechanism of experience-dependent synapse formation from their figures and discussions. Instead this literature focuses our attention solely on experience-expectant synaptic change. The advocacy literature zeros in on the windows of opportunity to the exclusion of life-long learning.
Numerous researchers in the area of brain development and early childhood have pointed to this deficiency in the early childhood advocacy literature. Greenough, a member of the committee that authored N2N, was himself critical of over-emphasizing experience-expectant at the expense of experience-dependent brain changes. He was even critical of how the topic was treated in N2N: “I have opposed those who argue that the human brain undergoes a ‘critical period’ between ages 0 and 3 after which it is too late to benefit from experience. I was a member of the committee that wrote the NAS/NRC report ‘From Neurons to Neighborhoods: The Science of Early Childhood Development,’ that took this view.” [xii]
Sir Michael Rutter, the British psychiatrist well-known for his research on adopted Rumanian orphans, has also written that claims about the singular importance and life-long effects of early experiences rests on a misappropriation of neuroscientific findings.[xiii] His view is that such claims are based on a misleading extrapolation of findings on experience-expectant development – synaptic blooming and pruning – to development in general where experience-dependent synapse formation also plays a substantial role.
My point here is not to cite authorities. Early childhood advocates could generate their own opposing list of authorities. The point is that the neuroscience and behavioral science research communities are not in agreement about the significance of windows of opportunity. Some take particular exception to how neuroscientific findings are used as evidence in the policy-advocacy literature.
Conclusion
Based on this brief review of the neuroscience claimed to be relevant to the policy potential of windows of opportunity, I would suggest policy makers be aware of the different emphases inherent in discussions based on Nelson’s original Figure 1 and those in the advocacy literature that use Figure 2. Omitting experience-dependent brain change from the discussions can skew our consideration of not only what might be prudent early childhood policy, but also of educational policy in general. Biologically constrained windows of opportunity are at best only half of the development and education story.
Of more concern is the contrast between the scholarly and advocacy claims about our knowledge of what experiences matter and when. On the scholarly side, Thompson and Nelson tell us we know little about what particular experiences matter. There may be windows of opportunity, but neuroscience cannot tell us how to exploit them. On the advocacy side, the claim is that specific experiences influence specific circuits at specific times, although most of the details and particulars remain vague. Instead we are offered the insight that neuroscience supports our common sense notions about parenting, education, and rehabilitation. It would be disappointing if all neuroscience provides to policy makers is support for common sense generalizations of breathtaking generality. One would hope that the science would allow us to move beyond common sense.
Common sense resides in the minds of the beholders, minds saturated by our own social and cultural biases. The value of scientific evidence is to challenge and move beyond received opinion and bias. Maybe campaign journalism has given way to campaign neuroscience. If so, we can understand why. As the psychologists tell us, neuroscience has a seductive appeal.
________________________________________
[i] https://www.whitehouse.gov/issues/education/early-childhood
[ii] http://www.nyas.org/Publications/Detail.aspx?cid=98de20dc-0de3-4bfe-9a20-ad07ab4a99ce
[iii] Weisberg, D.S., Keil, F.G. and Goodstein, J. (2008). The seductive appeal of neuroscience explanations. Journal of Cognitive Neuroscience 20(3): 470-477.
[iv] Shonkoff, J. P. and Phillips, D.A. (Eds). (2000). From Neurons to Neighborhoods: The Science of Early Childhood Development, Committee on Integrating the Science of Early Childhood Development. Board on Children, Youth, and Families National Research Council and Institute of Medicine National Academy Press Washington, D.C.; p. 188.
[v] Huttenlocher, P.R. (1979). Synaptic density in human frontal cortex – developmental changes of ageing. Brain Research.163: 195-205 and Huttenlocher, P.R and Dabholkar, A.S. (1997). Regional differences in synaptogenesis in human cerebral cortex. Journal of Comparative Neurology, 387, 167 – 178.
[vi] For the papers from this historic research program see Hubel, D.H. and Wiesel, T.N. (2004). Brain and Visual Perception: The story of a 25-year collaboration. Oxford: Oxford University Press.
[vii] Greenough, W.T, Black, J.E., and Wallace, W.S. (1987). Experience and brain development. Child Development, 58, 539 – 559.
[viii] For an example from the UK see http://www.centreforum.org/assets/pubs/parenting-matters.pdf and from Canada http://eys3.ca/en/report/download-early-years-study-3/. For examples in the science news see Bardin, J. (2012). Neurodevelopment: Unlocking the brain. Nature 487: 24 – 26 and Thomson, Helen. (2014). Reawakening the brain’s inner child. New Scientist 221(2951): 6 – 7.
[ix] National Scientific Council on the Developing Child (2007). The Timing and Quality of Early Experiences Combine to Shape Brain Architecture: Working Paper #5. http://www.developingchild.net.
[x] Thompson, R.A. and Nelson, C.A. (2001). Developmental science and the media: Early brain development. American Psychologist, 56(1), 5-15.
[xi] Rakic, P., Bourgeois, J-P, Echenhoff, M.F, Zecevic, N, and Goldman-Rakic, P.S. (1986). Concurrent over production of synapses in diverse regions of the primate cerebral cortex. Science, 232, 232 – 235.
[xii] Greenough, W.T. (1997).We can’t focus just on ages 0-3. APA Monitor, 28(11):19, November, 1997. Quotation can be found at APA Media referral Greenough.docarchives.library.illinois.edu
[xiii] Rutter, M. (2002) Nature, nurture, and development: From evangelism through science toward policy and practice. Child Development, 73(1), 1 – 21.



