

9:00 am EDT - 3:15 pm EDT
Past Event
9:00 am - 3:15 pm EDT
1775 Massachusetts Ave., NW
Washington, DC
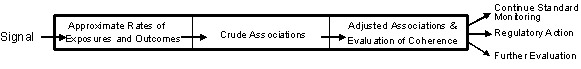
On September 21, the Engelberg Center for Health Care Reform hosted a one-day expert workshop to discuss the most effective and efficient ways to carry out the signal refinement step of active medical product surveillance. For the purposes of the meeting, signal refinement was defined as the step of active surveillance after signal generation, when a potential association between a medical product and health outcome is identified, and before signal evaluation, where formal epidemiological analyses are implemented. Potential steps in signal refinement are shown in the diagram below.
Experts from academia, the private sector, and FDA discussed several topics including:
Two hypothetical scenarios helped to guide discussion about signal refinement data and methodological needs:


Keon L. Gilbert, Keesha Middlemass, Nicol Turner Lee, Gabriel R. Sanchez, Rashawn Ray
March 5, 2026

Wendell Primus, Kristi Martin
March 4, 2026

Richard G. Frank, Kristi Martin, Rachel Sachs
March 2, 2026