High-profile shortages of amoxicillin, cancer drugs, and medications to treat attention deficit hyperactive disorder (ADHD) have put drug shortages in the headlines over the past year. Less visible but equally alarming have been shortages of lifesaving emergency drugs such as atropine and epinephrine. Many of these shortages are persistent.
These shortages throw into question the reliability of the U.S. drug supply chain. They also highlight how shortages can undermine patient care, endangering patient health and lives through delays in treatment, rationing, potential substitution with less effective alternatives, and increased risk of medication errors. Such shortages cost patients time and cause untold anxiety and flare-ups of underlying conditions.
The severity of drug supply disruptions has prompted the Biden administration and Congress to energetically search for solutions. Many policy solutions have been offered, including stockpiles, onshoring, advanced manufacturing, changes in Medicare reimbursement, greater transparency, and more. But how should we think about which policy ideas are the best? Given the number and variety of options, it is critical to determine which strategies are both effective and cost-effective. Without a strategic approach that recognizes different causes of shortages, we risk implementing expensive fixes that do little to make the U.S. drug supply chains more reliable.
In this paper, I describe a four-part test for assessing whether policy solutions are likely to accomplish improvements in supply chain reliability and whether they would do so cost-effectively. I also provide some background information to help understand different types of shocks that trigger shortages and the factors that prevent supply chains from withstanding those shocks. I do so for a range of types of supply disruptions. I then apply the four-part test to different types of policies that are being proposed to address the shortages, including transparency, buffer inventories, domestic manufacturing, advanced manufacturing, and reforms to hospital payments.
A four-part test for potential drug shortage solutions
Designing effective policy solutions calls for a full understanding of the problem to be addressed, as well as a consideration of unintended consequences and cost-effectiveness. Building on these general concepts of policy evaluation, I propose a four-part test for drug shortage solutions:
- Does the policy address the actual cause(s) of shortages? This is perhaps the most important question to ask when assessing a potential policy solution. Without properly mapping the solution to the problem, the solution may fall short on effectiveness, cost-effectiveness, or both.
- Can the policy work by itself or does its effectiveness depend on presence of other policies? Policymakers should consider whether additional policy changes are necessary for the proposed policy to have the desired effect.
- Does the policy anticipate and limit potential unintended consequences? Policy changes can change incentives for market participants or can enable those participants to act on existing incentives. To assess potential unintended consequences, it is important to consider how incentives of different market participants may be affected through the policy change.
- Is the policy cost-effective relative to alternatives? Building reliability into supply chains is costly – the more reliable we want supply chains to be, the more we will have to pay for it. Policymakers should assess the policy’s relative cost-effectiveness, especially considering the enormity of the U.S. drug supply chains.
The drug shortage problem(s)
In this section, I describe a general framework for how shortages can arise and then present how existing persistent shortages differ from potential shortages.
General framework for how drug shortages arise
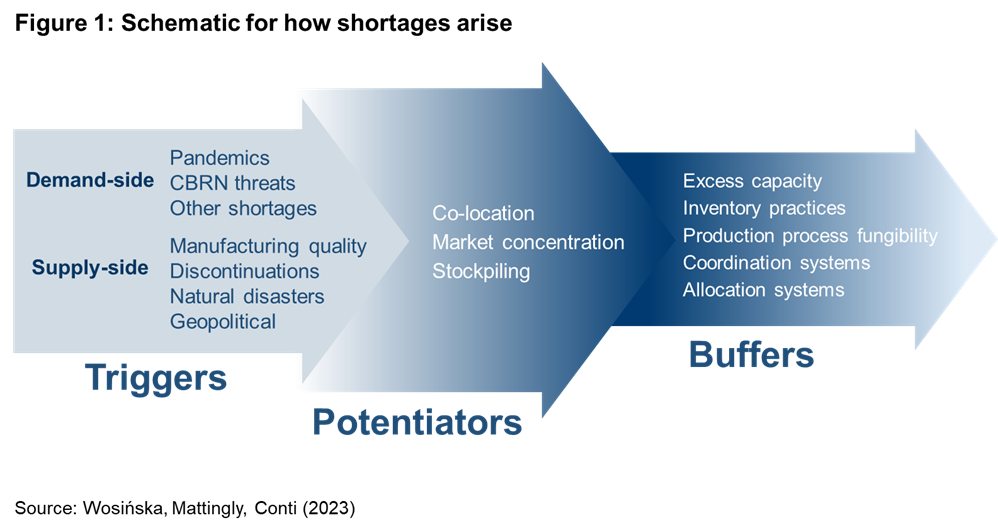
Shortages occur when supply chains cannot quickly adjust to demand shocks or cannot respond to disruptions to production or distribution of their products. In this section, I describe the vulnerability framework referenced in Figure 1, which categorizes shock types that may trigger drug shortages, as well as the factors that may enhance (or potentiate) the size of the shock and the buffers that may absorb or minimize it.
Demand shocks can occur for several reasons. One reason is a rapid increase in disease prevalence. For example, COVID-19 rapidly increased demand for ventilator drugs and the post-pandemic rise in respiratory diseases drove demand for amoxicillin. A demand shock can also be a drastic change in how a drug is used, as has been the case with the increase in the use of GLP-1 inhibitors for weight-loss. Demand can also spill over from a drug in shortage to another drug that might serve as substitute. Chemical, biological, radiological, and nuclear (CBRN) threats for which the government prepares could also cause major demand increases for medical countermeasures.
Disruptions to the supply chain can also occur for many other reasons, from manufacturing quality problems, natural disasters, and manufacturers discontinuing select products in their portfolio to disruptions in international trade due to geopolitical conflicts. These disruptions can occur at any stage of the production process, from raw materials to production of active pharmaceutical ingredients (API), inactive but critical ingredients, finished dosage form of a drug, and delivery mechanisms such as syringes.
But not every one of these shocks triggers a shortage. The extent to which a meaningful shortage results depends on the size of the resulting shock and the buffers that may exist.
Shocks often vary in size for reasons that cannot be controlled (e.g., size of a hurricane), but shock magnitude can also be determined by how the market is structured or operates. For example, a single facility may represent a large share of product sales, or manufacturing facilities may concentrate in one geographic area, making them vulnerable to a single shock like natural disaster or geopolitical instability. Structural dynamics can also affect the shock size through panic buying – uncertainty, low prices, ease of returns, and lack of allocation mechanisms can lead buyers to stockpile a drug at risk of shortage, with that precipitating or deepening the shortage.
A shortage ultimately results if a shock cannot be properly buffered. Buffering strategies can include dual sourcing, excess capacity, and reliance on manufacturing lines fungible enough to accommodate different types of products. Buffering strategies also include various inventory management practices: stockpiles set aside for times of emergency or buffer inventories where inventory levels in the supply chain are high enough so they can absorb greater shocks. Allocation mechanisms and coordination systems can also minimize the harm that results from shortages.
Characteristics of existing persistent drug shortages
Historically, shortages in the U.S. have been concentrated with generic sterile injectable drugs administered in hospitals and clinics. These drugs include baseline cancer therapies, intravenous (IV) nutrition, IV antibiotics, crash cart drugs to revive trauma patients, morphine, and saline. Figure 2 shows a recent snapshot in time, with 63% of all drugs in shortage attributed to generic sterile injectables. This high percentage continues to follow historical patterns for with 73% in 2011 and 63% between 2013-2017 (both numbers including an unknown but likely small number of branded drugs).
Manufacturing quality problems have persistently topped the list of reasons for drug shortages, representing 56% in 2011, 62% between 2013-2017, and 46% in 2022. Anecdotally, manufacturing problems disproportionately affect finished dosage form (FDF) facilities making generic sterile injectable drugs, and many large facilities are in the United States and Europe. The U.S. leads GSI production with over 40% of overall production volume.
Other causes of shortages – natural disasters, discontinuations, upstream supply disruptions, and demand increases – have followed manufacturing quality in varied order, depending on the year. To the extent there is a pattern to these causes, it is the increased frequency of demand-driven shortages that began with the pandemic, with shortages in that category reaching an all-time high in 2022 of 29%.
Notably, no shortages in the last 20 years appear to have been caused by export restrictions related to geopolitics, even during the pandemic.1
Sterile injectable drugs are particularly vulnerable to shortages because of the high rate of manufacturing quality disruptions they experience, coupled with inability of supply chains to absorb many such shocks.
The manufacturing quality disruptions are a result of market dynamics that start with hospital reimbursement mechanisms that incentivize hospitals to use the lowest priced drug available. These price pressures, coupled with inconsistent FDA surveillance, create a dynamic for manufacturers where there is little room for and return on investing in facilities, staffing, and oversight.
Generic drugs that are formulated as tablets or capsules also face similar price pressures from pharmacies, but they are less vulnerable to shortages because they face a different manufacturing environment and market structure. Manufacturing of those products is less complex, not requiring specialized facilities with employees following complex manufacturing processes and controls. Generic oral dose product markets are also less concentrated than sterile injectable ones, and there is more fungibility in oral dose production lines that can also be ramped up faster.
The shortage vulnerability of branded products, including injectable ones, also differs from that of generic injectables. Although branded sterile injectables face an equally, if not more, complex manufacturing environment, stable demand limits the need for switching between products on a line. Additionally, high margins earned by their products provide manufacturers with strong incentives to invest in trying to prevent disruptions to those products. Branded manufacturers have a greater incentive to invest in quality systems and to maintain spare capacity in case production unexpectedly must shut down. When production disruptions of this kind occur, they tend to resolve faster.
It is also worthwhile to highlight that two drug classes are responsible for much of the recent uptick in demand-driven shortages. On the one hand is the increase in demand for GLP-1 inhibitors, in shortage because of a significant increase in demand for off-label weight loss use. Given their production complexity, expansion of manufacturing capacity is lagging demand. Another prominent set of demand-driven shortages is the largely generic oral-dose group of drugs to treat ADHD. ADHD medications are controlled substances and although simpler to produce, the supply response is constrained by the aggregate and manufacturer-specific quota established by the Drug Enforcement Administration (DEA).
Characteristics of potential drug shortages
Without structural changes to how generic sterile injectable markets function, we are unlikely to see a change in the persistence of generic sterile injectable drugs. It is therefore encouraging to see Congress exploring policy ideas directly aimed at those shortages.
But pandemics, natural disasters, and export restrictions can also challenge supply chains. The question is: which supply chains would such shocks affect and to what extent?
A major geopolitical conflict could compromise many supply chains, potentially ones quite different from those currently at high risk of shortage. Such a conflict could also compromise production sites along many different stages of production, beyond the more limited set of FDF sterile injectable facilities that are at the heart of most current shortages. For these reasons, the location of API production has caught the attention of policymakers. But the U.S. government’s visibility falters beyond that, even though the exposure to countries with higher geopolitical risk is greater in the upstream supply chains.
One area that is particularly nontransparent to the U.S. government is the supply chain for precursors of API.2 A typical API will combine 2-5 starting materials with the help of so-called support materials. These support materials may include 1-3 catalysts, 3-10 solvents, and in some cases enzymes. An API may require several dozen inputs, with more complex APIs typically requiring more steps and therefore more starting materials to assemble the different intermediates that feed directly into the final API.
Government’s visibility is also lacking in the supply chain for excipients, the inactive ingredients that often make up 90% of a drug’s volume. “Inactive” is somewhat a misnomer because excipients help guarantee stability and bioavailability of the API, determine the texture and taste of the drug, and the rate at which it dissolves or binds. A given drug may typically have between 3 and 10 excipients, many of them not readily substitutable. Oral solid dose products typically have more excipients than injectables because they need excipients to help with the bioavailability of the API, while injectable products have almost complete bioavailability. Magnesium Stearate is perhaps the most common excipient, appearing in over 36,000 drug products.
In some ways, vulnerability of supply chains to natural disasters mirrors that of geopolitical risks: they can affect any part of the supply chain and there is similarly limited visibility upstream, limiting opportunities for a comprehensive vulnerability assessment beyond FDF and API. The difference is that supply disruptions due to natural disasters are likely more localized than geopolitical risks.
In turn, pandemics and CBRN threats primarily result in demand shocks for a relatively narrow set of products. For some pathogens, drugs and vaccines are known, but for others, drugs, tests, or vaccines may not exist. These threats also have the possibility of creating supply chain disruptions for which previous discussion offers a guide.
Table 1 summarizes the trigger characteristics across the most common trigger types.
Identifying appropriate policy solutions
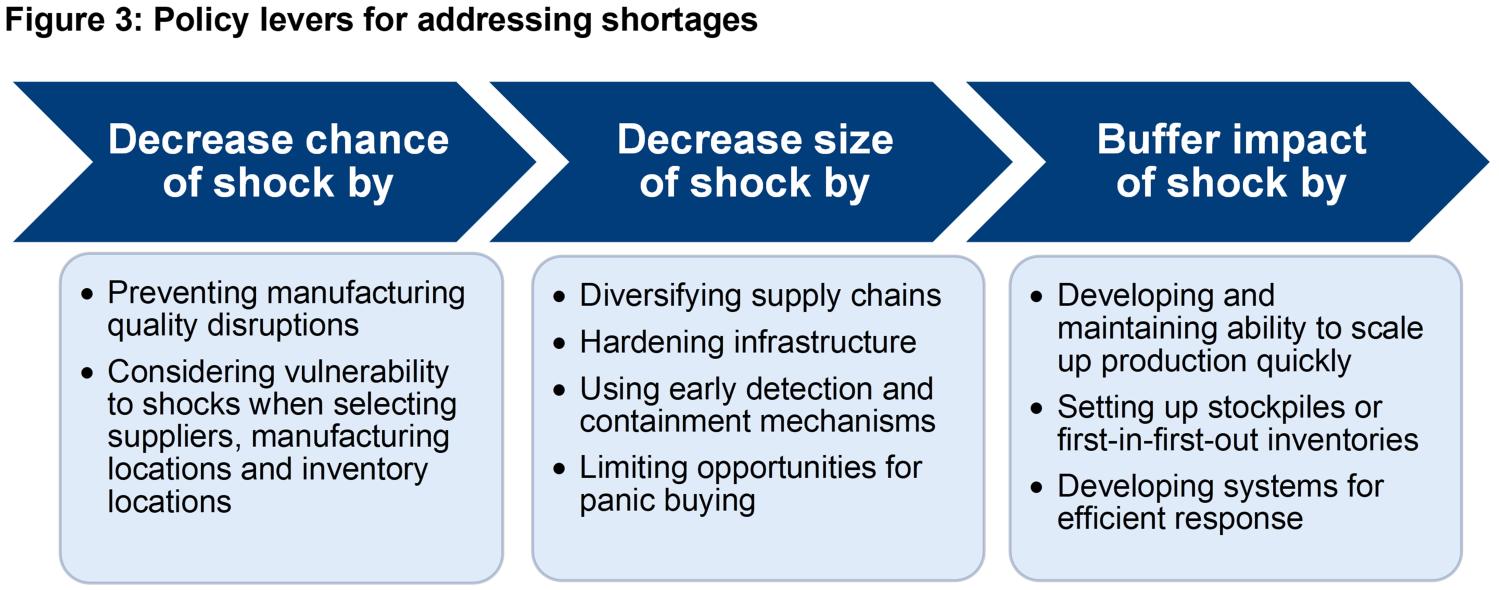
The above discussion about various causes of drug shortages suggests that different strategies are needed to address existing and potential shortages. It also suggests that an effective strategy should consider prevention mechanisms that lower the chance of and the likely size of a specific shock type. Figure 3 characterizes the three major levers policymakers have in addressing shortages.
An effective strategy is multipronged, addressing the three levers to varying degree. The most effective and most cost-effective combination of Figure 3 levers will depend on the nature of the shortage and the relative effectiveness and cost-effectiveness of the three types of levers. Policymakers can arrive at the right combination by identifying the actual problem, avoiding incomplete solutions, addressing unintended consequences, and considering return-on-investment – the four-part test I lay out earlier in this article.
Table 1 presents a simplified output of such an analysis for key shortage triggers.
Refining drug policy solutions
To help illustrate how I arrived at the policy solutions listed in Table 1, I apply the four-part test to the baseline version of several policy proposal categories: transparency, stockpiling, domestic manufacturing, advanced manufacturing, and hospital payment reforms. I show here how the tests might play out in different cases and under different circumstances.
For exposition purposes, the discussion below may not follow the order of the four-part text. In addition, the analysis here represents key aspects but is by no means a complete assessment of these policies. I also note that the criticisms included here do not imply that the policies have no standing, but rather that there are important aspects that policymakers need to consider and address.
Transparency
Transparency initiatives are often invoked among reforms to address shortages. They cover a gamut, from government’s visibility and buyers’ visibility into supply chains, to whether government shares information in enough detail or in a readily available manner.
In the right context, transparency can help “assess, mitigate, prepare for, and respond to risks of medical product shortages.” For example, reforms in 2011 and 2012 gave FDA greater visibility into supply disruptions, enabling the agency to coordinate a response. With greater visibility into supply chains, the federal government can assess which supply chains are most vulnerable, prioritizing those for intervention. With more detail about what triggered a particular shortage, buyers can get feedback on which manufacturers are more reliable. With more information about reliability of manufacturers, buyers can shift purchasing accordingly.
However, transparency by itself will not accomplish anything if recipients of the information have no incentive to act on it. Releasing more information about reliability of manufacturers is not likely to succeed without a change in incentives to hospitals that currently focus on the lowest cost generic option. In fact, much relevant information about reliability of manufacturing already exists, but it is underutilized because hospitals are reluctant to buy anything but for the lowest price available.
In the wrong context, transparency can have unintended consequences. Take early warning systems for shortages. To a hospital, an early warning signal of shortage is a signal to start stockpiling, precipitating the shortage. Similarly, placing country of origin on a retail prescription label could backfire with higher rates of nonadherence if the only choice that a patient has at a pharmacy is whether to pick up a prescription or not, unlike in a retail store where a consumer has options to choose from multiple versions of the same product.
A proper assessment of transparency initiatives would involve applying the four-part test to each transparency proposal separately, paying particular attention to the intended audience and their ability and incentives to act on the information.
Stockpiling and buffer inventory
Holding higher levels of inventory can buffer against the adverse impact of a shock, no matter the shock’s etiology. This common application makes stockpiling and buffer inventories a potentially attractive policy solution.
Stockpiles and buffer inventory can be important because not every shock can be prevented. But one cannot buffer every drug product, and with most shortages lasting over a year, it would be prohibitively expensive to buffer a shortage with reserve stock that will last until the supply shortage is resolved. For this reason, it is important to prioritize which products are stockpiled, considering not only whether these products are medically necessary but also whether their supply chains are vulnerable. For other products, such as highly used large volume products like saline, stockpiling the FDF product may not be practical, requiring an alternative set of buffering strategies.
Stockpiling or buffer inventory proposals are also incomplete if they do not address panic buying that ensues at the first sign of a potential shortage. If the government creates a buffer inventory and then releases it, a “bank run” on the product is likely to result. Currently such “bank runs” are uneven, usually with the large hospital systems able get to the product first. For this reason, any government funded stockpile should have allocation mechanisms in place, even if they are simply historical allocations. Otherwise, providers most likely to currently suffer from shortages will continue to suffer.
Lastly, it is important to acknowledge that stockpiles or buffer inventories are a form of insurance in case shocks cannot be prevented or minimized. It may be near impossible to prevent a natural disaster, but on the other hand, quality lapses, which are the primary reason behind shortages, are not only possible but important to address. Without addressing the root cause of manufacturing quality problems, products not made to specification may and can reach patients during non-shortage times, potentially causing harm.
Domestic manufacturing
Domestic manufacturing is offered as a solution to drug shortages perhaps more often than any other policy proposal. This policy appears steeped in concerns over the loss of U.S. manufacturing base, coupled with concerns over supply chain exposure to geopolitical risks that were starkly underscored during the pandemic.
But for all the attention it attracts, the standard “we need to bring manufacturing back to the U.S.” proposal is challenged on three of the four parts of the test: addressing the right problem, completeness of the solution, and assuring cost-effectiveness.
On the right-problem front, domestic manufacturing can be a solution for addressing geopolitical risks. But proposals tend to focus on either the finished dosage form through “buy-American” policies or onshoring of API production. It is true that much FDF and API manufacturing has moved offshore, but does subsidizing U.S. production of drugs substantially improve supply chain reliability when those companies still rely on inputs from China? Without addressing that reliance, such proposals are incomplete, presenting poor return on investment for taxpayers.
Domestic manufacturing also does not address the drivers of persistent shortages of generic sterile injectable drugs. These shortages are not a result of geopolitics or domestic versus foreign quality differences. Rather, these shortages result because hospitals, the buyers of these drugs, do not reward manufacturing quality and reliability, leading to manufacturing quality shortfalls in domestic and foreign facilities alike.
The pricing market pressures driving existing shortages also highlight the need to attach strings on quality to domestic manufacturing investments or else such investments will falter. Any government subsidies to bolster domestic manufacturing in response to geopolitical risks should consider other types of shocks, such as natural disasters. For example, we might have a lot of idle capacity in Puerto Rico, but that is also an area vulnerable to hurricanes.
Domestic manufacturing as a solution is also challenged on the cost-effectiveness front. As described in the section on potential shortages, the pharmaceutical industry and the chemical industry that feeds the key starting materials for drugs may have extensive exposure to countries with high geopolitical risk. To lower this risk, a proper risk mitigation would make diversification through friend-shoring and near-shoring an integral part of U.S. government strategy.
Advanced manufacturing
Because outdated production technologies contribute to the high rate of manufacturing quality problems, the use of advanced manufacturing technologies – continuous manufacturing in particular – is often proposed as a solution that could lower the likelihood of manufacturing disruptions.
These proposals fail to recognize just how strongly economic forces driving shortages of generic sterile injectable drugs work against adoption of such tools. The low margins on drugs at greatest risk of shortage mean that the federal government would have to heavily if not fully subsidize these technologies. And even with full subsidies of installation costs, these technologies may not translate well into reliability in an environment where the unstable nature of the demand can lead to 20-30 products being run on a single line over a course of a year, leading to frequent switchovers that are at the heart of many of the existing disruptions.
To the extent the federal government were to subsidize technology improvements, it should consider whether other, potentially much simpler technology solutions may be more cost-effective. There may be appropriate cases for using advanced manufacturing but tying a significant share of subsidies to advanced technologies would limit the reach of widely-needed infrastructure investments.
Hospital payments for buying reliably
Changing hospital payments to encourage hospital pharmacy procurement from more reliable manufacturers directly addresses the root cause of persistent shortages of generic sterile injectable drugs. By modifying how CMS pays for such generic sterile injectable drugs, CMS can steer hospitals away from their heavy emphasis on price and towards reliability of supply.
But even though this policy area addresses the root cause of the problem, how it is designed would influence its cost-effectiveness. To the extent that CMS were to adopt add-on payments, the effectiveness of such an add-on payment in preventing shortages would depend on CMS’s (or FDA’s) ability to identify which manufacturers are reliable. The better the predictive power of such measures, the greater the impact of an add-on payment program tied to such list of reliable manufacturers. If those measures are not reliable, CMS would be increasing government spending without making a difference on the shortage front. This in turn would translate into poor taxpayer return-on-investment.
Changing hospitals reimbursement for generic sterile injectable markets would also likely falter without changes to Medicaid inflation rebates for outpatient generic drug markets with a large 340B presence (e.g. generic cancer drugs recently in shortage). Medicaid inflation rebates neutralize price increases in the Medicaid and the 340B market segment. But without the ability to pass on cost increases, however reasonable, manufacturers will have little incentive to make the investments necessary to differentiate themselves on manufacturing reliability, which is the entire premise behind payment reforms to address shortages.
Conclusion
Our drug supply chains are not as reliable as we expect them to be, resulting in disruptions in medical care and causing patient harm. But little progress will take place unless there is a systemic change in the economic dynamics and the misaligned incentives that exist in the marketplace. Because economic dynamics are at play, there is an important role for the U.S. government to drive change.
But building reliability into supply chain does not come for free. Between the enormity of the drug supply chains and the limited resources that Congress is likely to appropriate toward solving drug shortage problems, government intervention can easily become a feel-good strategy that does little to improve supply chain reliability where it matters most. Policymakers can avoid such fate by properly mapping solutions to the underlying problem, avoiding incomplete solutions, considering return-on-investment, and addressing unintended consequences. This article presents a guide to the nature of shortages and ways to accomplish the best outcome we can obtain with limited resources.
-
Acknowledgements and disclosures
This work was supported by Arnold Ventures. I would like to thank Richard Frank, Rena Conti, Erin Fox, Tim Manning, and Amy Goldstein for helpful comments. I would also like to thank Caitlin Rowley, Chloe Zilkha, and Chris Miller for their publishing support.
The Brookings Institution is financed through the support of a diverse array of foundations, corporations, governments, individuals, as well as an endowment. A list of donors can be found in our annual reports published online here. The findings, interpretations, and conclusions in this report are solely those of its author(s) and are not influenced by any donation.
-
Footnotes
- Interview with Erin Fox, University of Utah Drug Information Service.
- The API and excipient discussion that follows is based on an interview with Carlo di Notaristefani, former head of manufacturing at Teva and a former consultant to Operation Warp Speed.
The Brookings Institution is committed to quality, independence, and impact.
We are supported by a diverse array of funders. In line with our values and policies, each Brookings publication represents the sole views of its author(s).




Commentary
Drug shortages: A guide to policy solutions
March 13, 2024