As the 2016 suite of new car models makes evident, electric vehicles are finally gaining real traction in the market. At the turn of the 20th century, more than one quarter of all cars in the United States were electric, yet the electric car had all but vanished by the 1920s. This disappearance was largely due to the insufficient range and power of electric car batteries compared to gasoline engines. Furthermore, electric cars were significantly more expensive than their gasoline counterparts. These same complaints are still heard today, even though battery technology has certainly improved over the last century. Much research and development is being done on battery technology to improve performance while ensuring that batteries are lightweight, compact, and affordable.
So, what are the newest innovations in battery technology, and what do such advances mean for the electric vehicle market?
-
Lithium-ion batteries
Lithium-ion batteries (LIBs) are currently used in the majority of electric vehicles, and it’s likely that they will remain dominant into the next decade. Several manufacturers, including Tesla and Nissan, have invested heavily in this technology. In LIBs, positively charged lithium ions travel between the anode and the cathode in the electrolyte. LIBs have a high cyclability – the number of times the battery can be recharged while still maintaining its efficiency – but a low energy density – the amount of energy that can be stored in a unit volume. LIBs have garnered a bad reputation for overheating and catching on fire (e.g. Boeing jets, Tesla cars, laptops), so manufacturers have not only worked to make LIBs more stable, but they have also developed many safety mechanisms to prevent harm if a battery were to catch fire.
The LIBs on the market today primarily use graphite or silicon anodes and a liquid electrolyte. A lithium anode has been the holy grail for a long time because it can store a lot of energy in a small space (i.e. it has a high energy density) and is very lightweight. Unfortunately, lithium heats up and expands during charging, causing leaked lithium ions to build up on a battery’s surface. These growths short-circuit the battery and decrease its overall life. Researchers at Stanford recently made headway on these problems by forming a protective nanosphere layer on the lithium anode that moves with the lithium as it expands and contracts.
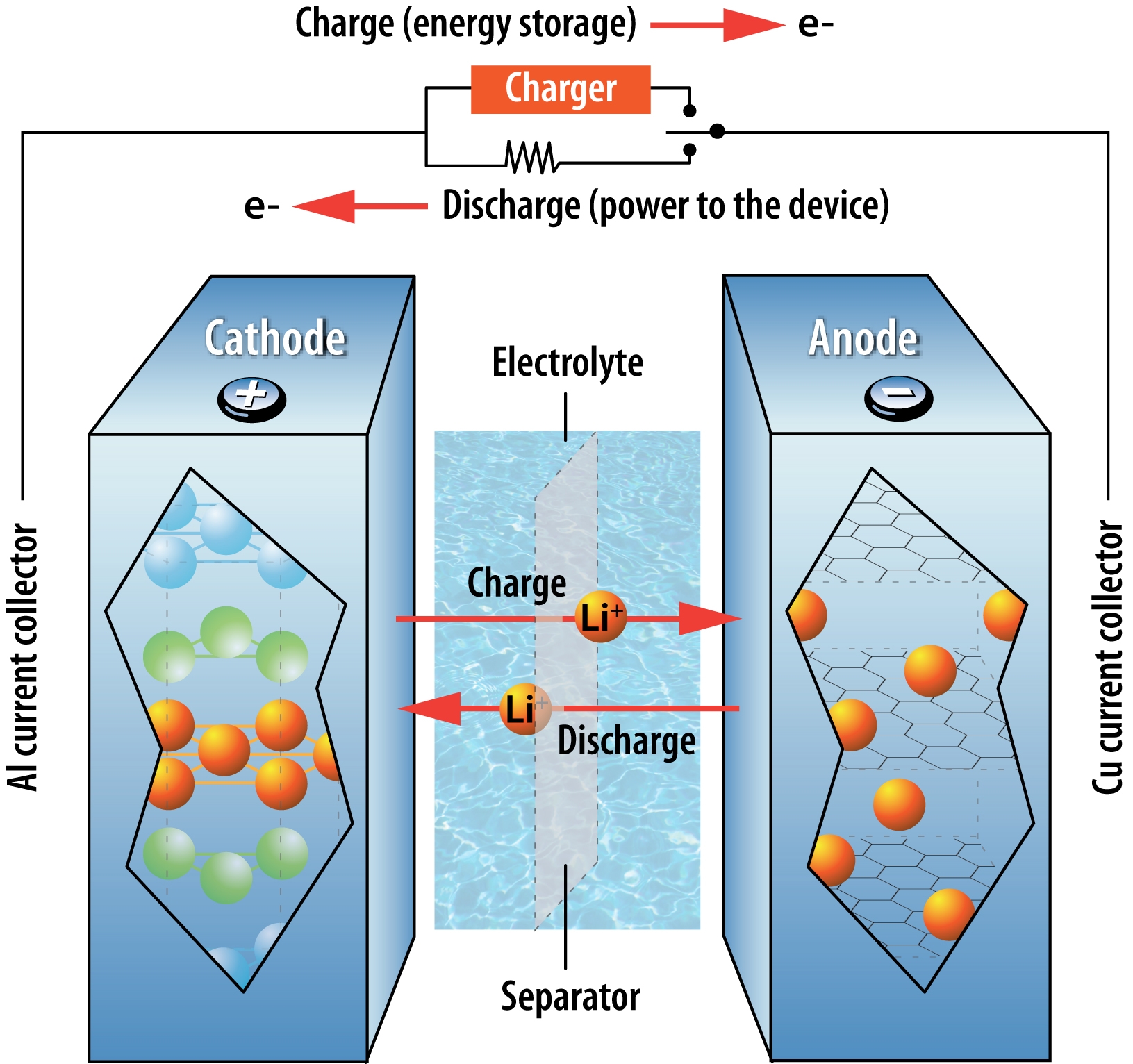
Movement of lithium ions and electrons in a lithium-ion battery during charging and use.
Source: Argonne National Laboratory. Used under Creative Commons license.
Solid state batteries
Solid-state batteries have solid components. This construction provides several advantages: no worry of electrolyte leaks or fires (provided a flame-resistant electrolyte is used), extended lifetime, decreased need for bulky and expensive cooling mechanisms, and the ability to operate in an extended temperature range. Solid-state batteries can build off of the improvements made in other types of batteries. For example, Sakti3 is trying to commercialize solid-state, LIBs with funding from General Motors Ventures. Other auto manufacturers, such as Toyota and Volkswagen, are also looking into solid state batteries to power their electric cars.
Aluminum-ion batteries
Aluminum-ion batteries are similar to LIBs but have an aluminum anode. They promise increased safety at a decreased cost over LIBs, but research is still in its infancy. Scientists at Stanford recently solved one of the aluminum-ion battery’s greatest drawbacks, its cyclability, by using an aluminum metal anode and a graphite cathode. This also offers significantly decreased charging time and the ability to bend. Researchers at Oak Ridge National Laboratory are also working on improving aluminum-ion battery technology.
Lithium-sulfur batteries
Lithium-sulfur batteries (Li/S) typically have a lithium anode and a sulfur-carbon cathode. They offer a higher theoretical energy density and a lower cost than LIBs. Their low cyclability, caused by expansion and harmful reactions with the electrolyte, is the major drawback. However, the cyclability of Li/S batteries has recently been improved. Li/S batteries, combined with solar panels, powered the famous 3-day flight of the Zephyr-6 unmanned aerial vehicle. NASA has invested in solid-state Li/S batteries to power space exploration, and Oxis Energy is also working to commercialize Li/S batteries.
Metal-air batteries
Metal-air batteries have a pure-metal anode and an ambient air cathode. As the cathode typically makes up most of the weight in a battery, having one made of air is a major advantage. There are many possibilities for the metal, but lithium, aluminum, zinc, sodium remain the forerunners. Most experimental work uses oxygen as the cathode to prevent the metal from reacting with CO2 in the air, because capturing enough oxygen in the ambient air is a major challenge. Furthermore, most metal-air or metal-oxygen prototypes have problems with cyclability and lifetime.
Batteries are often underappreciated when they work as designed, but harshly criticized when they don’t live up to expectations. The technologies highlighted above are by no means an exhaustive list of the developments that have been made. Electric vehicles will undoubtedly become more commonplace as batteries are improved. Advancements in batteries could not only transform the transportation industry, but they could also significantly affect global energy markets. The combination of batteries with renewable energy sources would drastically diminish the need for oil, gas, and coal, thereby altering the foundation of many economic and political norms we currently take for granted. We certainly don’t have to wait until the “perfect battery” is developed to recognize tangible improvements in performance. Despite the current shortcomings of batteries, the potential global impact that even relatively moderate improvements can have is astonishing.
Elsie Bjarnason contributed to this blog post.
The Brookings Institution is committed to quality, independence, and impact.
We are supported by a diverse array of funders. In line with our values and policies, each Brookings publication represents the sole views of its author(s).


Commentary
Five emerging battery technologies for electric vehicles
September 15, 2015