The international effort to achieve global COVID-19 vaccination goals faces a dilemma. Stakeholders in this space are at odds over how to treat intellectual property (IP) rights now that viable vaccines are on the market but are inaccessible to vulnerable populations in low- and middle-income countries (LMICS). A key aspect of this debate is whether to grant patent waivers for COVID-19 vaccines and therapeutics. We suggest looking beyond patent waivers with an innovative solution based on public-private partnerships (PPPs), an approach that could be more effective in combating the on-going COVID-19 pandemic and simultaneously help prepare LMICS for future health crises.
Background
Created in 1995, the World Trade Organization (WTO) provides a forum for member states to lower barriers to international trade. The WTO also serves as a legal and institutional framework for executing multilateral agreements related to the global trading system. One of these agreements is known as Trade-Related Aspects of Intellectual Property Rights (TRIPS). TRIPS governs the protection of IP rights, such as patents and trademarks. Technologies that prevent, contain, and treat COVID-19—the death toll from which exceeds 4.3 million people with over 200 million infected—are protected under TRIPS. This intersection of global public health and international trade regulations has spurred a debate as to whether an exception to TRIPS for COVID treatments is warranted. The two schools of thought on the patent waiver issue can be roughly characterized as follows:
- Pro-patent protection: The first school that patent protections on COVID-19 vaccines are necessary because pharmaceutical companies will otherwise be disincentivized to innovate and invest in vaccine research and development, and they will unfairly lose market share to competitors and adversarial nations such as China. This theory also that removing IP protections will not serve the intended objective of increasing vaccination rates as the developing world lacks the infrastructure and expertise to roll out effective domestic production. Advocates of patent protection argue that the WTO already allows countries to apply for “compulsory licensing,” which waives IP during emergencies such as the COVID-19 pandemic. Proponents of continued patent protection see voluntary commitments from industry, developed world governments, and large NGOs as a more effective means of addressing the problem.
- Pro-patent waiver: Conversely, others removing IP protections is a necessity as companies located in high-income countries hold most, if not all, of the COVID-19 vaccine IP and sell the vaccines to governments mostly in the developed world. According to this view, the price of these vaccines, combined with export restrictions and the inability of LMICs to manufacture their own vaccines at a lower price and without fear of litigation from patent holders, are among the main reason why vaccines are not reaching the world’s most vulnerable communities. They further that the compulsory license process is both time consuming and cumbersome and that providing basic medical services for these vulnerable communities should be prioritized over industry profits. Finally, a more diffuse global vaccine manufacturing architecture would be more effective and in line with health as a human right.
The patent waiver issue gained traction in response to the stark disparity in global health outcomes as COVID-19 vaccines and therapeutics were brought to market. Data from May 2021 indicates that “people living in G7 countries were 77 times more likely to be offered a vaccine than those living in the world’s poorest countries.” Data from the end of June 2021 reflects that “46% of people in high-income countries had received at least one dose of the COVID-19 vaccine compared with 20% in middle-income countries and only 0.9% in low-income countries.”
This global health inequity is in part due to high-income countries purchasing more vaccines than they need. For example, Canada has secured vaccine doses for 434% of its population. Another issue is vaccine price in relation to cost and LMIC’s purchasing power: One report states that Pfizer/BioNTech and Moderna have been charging governments up to 24 times the potential cost of production. Furthermore, Pfizer/ BioNTech are charging their lowest reported price of $6.75 to the African Union, yet one dose costs the same as Uganda spends per citizen on health annually. These incongruities represent an injustice to the world’s underserved populations, and they demand the development of innovative ideas regarding how to overcome price and access obstacles.
In October 2020, India and South Africa led a group of LMIC’s request to the WTO to waive certain TRIPS provisions. The request, modified as of May 25, 2021, asks for a three-year waiver of IP protection for products and technologies related to COVID-19 prevention, treatment, and containment. Normally, WTO protections for IP last around 20 years. The Biden administration is currently on board with the waiver, and the EU is open to negotiations. However, some EU member states like Germany remain steadfast in rejecting this idea, and the EU has proposed its own non-waiver plan. The WTO will likely take months deliberating this matter, and it usually renders decisions unanimously, though a TRIPS waiver would technically only require a three-quarters majority to pass. As it stands now, talks at the WTO stalled in late July with little progress and are now on hold for the summer holiday.
Solution
An optimal solution to the currently inequitable global distribution of COVID-19 vaccines requires more innovation than a temporary waiver of patents. A process is needed whereby LMICs can take some level of ownership over the manufacturing and distribution of critical vaccines and medicines without the bureaucratic red tape associated with compulsory licensing. We suggest that PPPs between pharmaceutical companies and relevant governmental ministries that are well-funded by access to the capital markets through impact bonds is a comprehensive, sustainable solution to the problem of achieving global vaccination goals.
A PPP can be defined as:
Co-operation of some sort of durability between specific public and private actors in which they jointly develop infrastructure, products, and services (including knowledge and dissemination of information) and share risks (financial and/or prestige), cost and resources, which are applied in the development and delivery process.
This solution has three essential components: first, identifying the incentives for the private sector to participate in the partnership; second, inducing the public sector to transfer some of its mission and responsibilities to the partnership; and third, access to capital markets.
As the current authors wrote in 2016: Private sector entities can profit from PPPs—especially with LMICs that present a new or unsaturated market for a wide range of a pharmaceutical company’s products. Increased brand recognition, increased market penetration, entry into new markets, preserving the existing customer base, gaining new customers, and garnering favorable status for introduction of new products are all attractive concepts for private sector partners. Relaxed barriers to market entry (e.g., tariffs and taxes) and access to LMIC raw data would also motivate a private sector entity to forge a relationship with public entities.
The public sector can be incentivized to formalize a PPP for pharmaceutical and vaccine-related issues like supply chain management, data capture and analysis, quality control, and inventory optimization. PPPs would assist in speeding up the scaling required to develop sufficient quantities of COVID-19 vaccines and medicines, and LMICs would be better prepared for future pandemics.
Access to the capital markets through “impact bonds” can provide a source of sustainable funds. Impact bonds work in a series of steps (see Figure 1. below): Investors purchase bonds and provide up-front risk capital to finance the program(s). Prior to issuance of the bonds, well-defined metrics leading to specific sets of outcomes for success of the partnership need to be negotiated. The progress toward fulfilling these outcomes will be monitored and rigorously measured by an independent organization at every stage.
When the partnership demonstrates that it has met its goals, the outcome payers—who can be public sector entities (i.e., Ministries of Health or Finance), the private sector, development banks, or combinations of all three—are contractually and legally required to repay the investors. The key advantage of this approach is the additional accountability for outcomes that investment brings. Investors’ interest in achieving measurable success provides a framework that incentivizes flexible and effective program implementation. Risk is transferred to the investor, and the focus on rigorously measured outcomes ensures that scarce donor funding is only used for tangible, verifiable outcomes. The metrics, goals, and outcomes must be uniquely crafted for each country in which the impact bond is issued. Ultimately, a successful PPP might lead to healthier populations, more robust and cost-effective national healthcare systems, and economic growth.
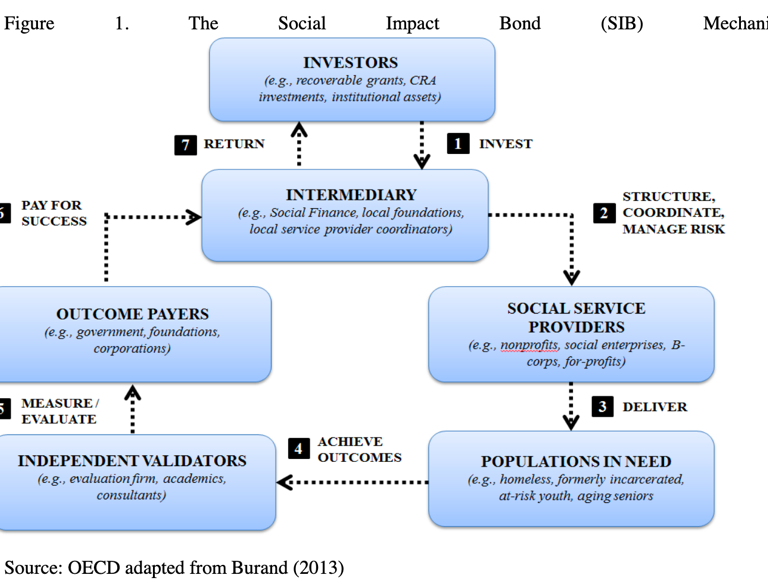
Source: Understanding Social Impact Bonds, OECD Working Paper, 2016
As Brookings Institution scholars wrote in a review of USAID’s PPPs: “On a conceptual level, public-private partnerships are a win-win, even a win-win-win, as they often involve three types of organizations: a public agency, a for-profit business, and a nonprofit entity. PPPs use public resources to leverage private resources and expertise to advance a public purpose. In turn, non-public sectors—both businesses and nongovernmental organizations (NGOs)—use their funds and expertise to leverage government resources, clout, and experience to advance their own objectives, consistent with a PPP’s overall public purpose. The data from the USAID data set confirm this conceptual mutual reinforcement of public and private goals.”
A case study is further illustrative of how PPPs play an integral role in pandemic-related solutions. Established in 2003, The U.S. President’s Emergency Plan for AIDS Relief (PEPFAR) is a U.S. government foreign aid program focused on controlling the HIV/AIDS epidemic in more than 50 countries. PEPFAR has saved millions of lives; experts note that PPPs played a key role in this effort, strengthening logistics, supply chains, and HIV lab practices:
PEPFAR’s Supply Chain Management System took advantage of private industry’s best practices in logistics, and a partnership with the medical technology company BD (Becton, Dickinson and Company) improved laboratory systems throughout sub-Saharan Africa. We found that setting ambitious goals, enlisting both global and local partners, cultivating a culture of collaboration, careful planning, continuous monitoring and evaluation, and measuring outcomes systematically led to the most effective programs.
Other examples of successful PPPs in global health include the Global Alliance for Vaccines and Immunizations (GAVI); the Global Fund to Fight AIDS, TB and Malaria; Global Alliance for TB Drug Development, Drugs for Neglected Diseases initiative (DNDi); International AIDS Vaccine Initiative (IAVI); Medicines for Malaria Venture; Harnessing Non-State Actors for Better Health for the Poor; and PPPs for Universal Health Coverage.
Conclusion
Patent waivers will not correct the lack of capacity in the majority of LMICs that is necessary to implement domestic production of vaccines. Cold chain infrastructure, logistics and data systems, robust supply chains (including access to the raw materials needed for disease testing and vaccine/medicine production), and storage and administration need to be developed. Finally, there is a desperate need to train and maintain a skilled workforce to permanently meet not only the ongoing challenges of the current pandemic and any future pandemic but also to build capacity and jobs in the biomedical sectors.
Implementing an impact bond-funded PPP to fully develop, manage, and sustain a vaccine and critical medicine supply/cold chain is the most promising path forward to broaden access to COVID-19 vaccines and therapeutics in LMICs. It’s an ambitious goal that requires cooperation among entities with disparate interests, but the current alternatives are not working. The patent waiver debate could yield fruit by perhaps streamlining TRIPS’ compulsory licensing process or by granting waivers to countries that have the capacity to make generics at lower cost. However, the core long-term problem for most LMICs will remain without engagement with the private sector’s expertise and access to capital markets. PPPs are the best way these countries will be able to strengthen their infrastructure, supply chain capacity, and technical expertise sufficiently and permanently in order to respond to pandemics effectively—a result that is required for global health security and equity.
The Brookings Institution is committed to quality, independence, and impact.
We are supported by a diverse array of funders. In line with our values and policies, each Brookings publication represents the sole views of its author(s).







Commentary
Innovation beyond patent waivers: Achieving global vaccination goals through public-private partnerships
August 31, 2021