In an interview with CBS news last week, new U.S. Surgeon General Vivek Murthy suggested marijuana can be helpful for some medical conditions, and that science should dictate the United States’ policy on the matter. However, under the current system, the Surgeon General’s wishes will largely go unfulfilled.
What stands in the way of a broad role for medical science in marijuana policy is the Controlled Substances Act. Under this law, marijuana is listed as a Schedule I substance, meaning it has a high potential for abuse and no current accepted medical use. Substances classified in Schedules II-V are still subject to varying degrees of control, but have a recognized medical use and may be dispensed with a prescription under certain circumstances. They are also subject to robust research, testing, and manufacture. For marijuana to enjoy the same benefits, it would need to be rescheduled.
So, in the tradition of ‘how a bill becomes a law,’ we hope to explain how a drug becomes a legally-defined less dangerous drug.
There are two ways by which the scheduling of marijuana can be changed: congressional action and administrative action.
Congress has the power to reschedule marijuana, either through new legislation specific to marijuana or through tailored amendments to the Controlled Substances Act. The first bill that proposed to move cannabis from Schedule I to Schedule II was introduced by Representative Stewart McKinney (R-CT) in 1981. Similar bills have been introduced perennially since then, most recently by Rep. H. Morgan Griffith [R-VA] (H.R. 4498), all of which died in committee. In 2011, Reps. Ron Paul (R-TX) and Barney Frank (D-MA) introduced a bill to remove marijuana from the schedules entirely (“de-scheduling”), which also died in committee.
President Obama has contended that rescheduling marijuana is a job for Congress, while others rightly argue the administration has the authority to do so unilaterally. It is ironic that the president, who is so often criticized for overreaching his authority, is shrinking from the administrative power that Congress has granted him.
So, how does administrative rescheduling work? It is not as easy as some in the marijuana advocacy community—and critics of the Obama administration’s position on this issue—would have you think. It is a complex process in which scientific, medical, policy and political forces have influence. Below is a flowchart that explains how rescheduling works.
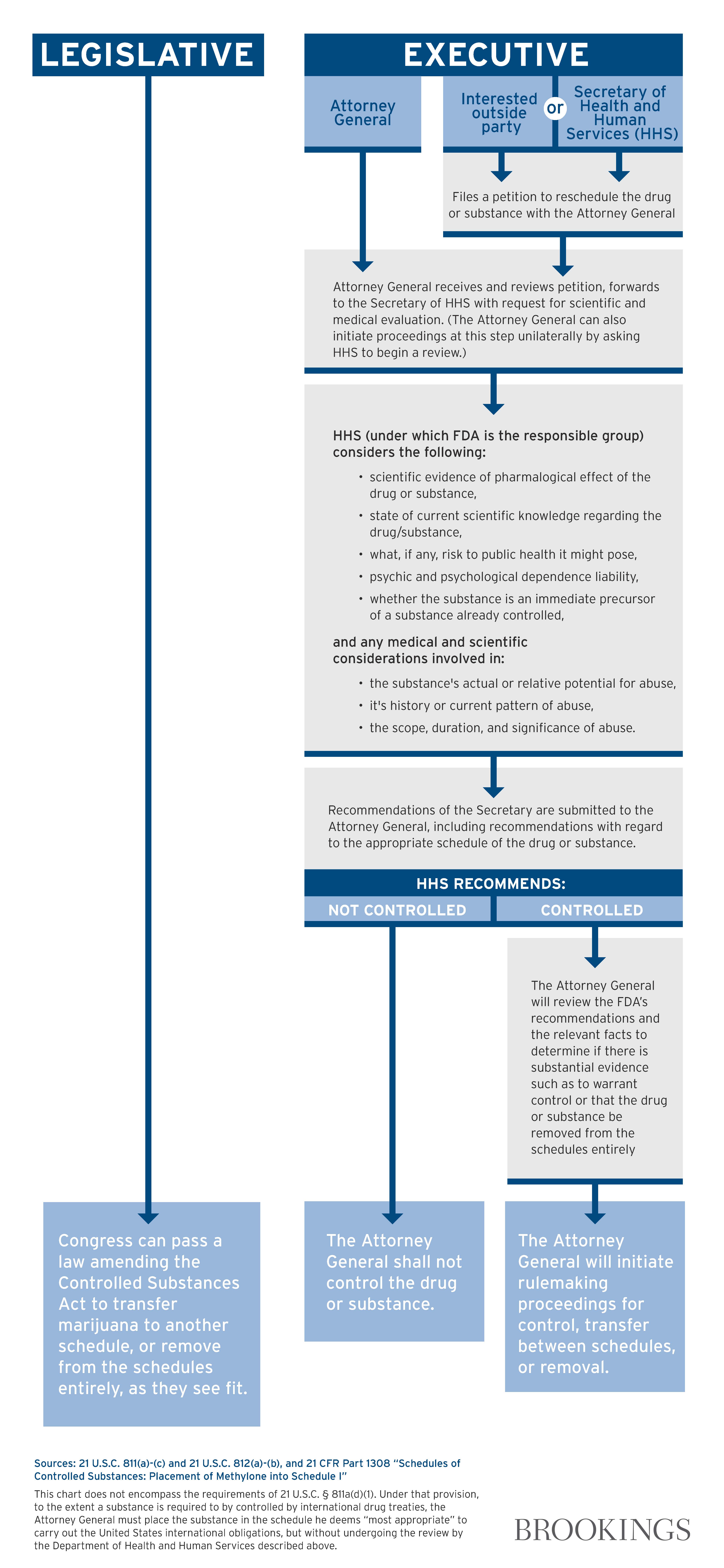
In a nutshell, administrative rescheduling begins when an actor—the Secretary of Health and Human Services or an outside interested party—files a petition with the Attorney General or he initiates the process himself. The Attorney General forwards the request to the HHS Secretary asking for a scientific and medical evaluation and recommendation, as specified by 23 USC 811(b-c). HHS, via the Food and Drug Administration conducts an assessment and returns a recommendation to the Attorney General “in a timely manner.” The Attorney General, often through the Drug Enforcement Administration, conducts its own concurrent and independent review of the evidence in order to determine whether a drug should be scheduled, rescheduled, or removed from control entirely—depending on the initial request in the petition.
If the Attorney General finds sufficient evidence that a change in scheduling is warranted he then initiates the first stages of a standard rulemaking process, consistent with the Administrative Procedures Act. During rulemaking and consistent with Executive Order 12866, if the White House—through the Office of Management and Budget’s Office of information and Regulatory Affairs—determines the rule to be “significant,” it will conduct a regulatory review of the proposed rule—a very likely outcome given the criteria in the EO.
The above process and the associated flow chart simply outline the structural procedures required to reschedule marijuana, but there are other concerns that surround rescheduling and we discuss a few below.
How do state actions affect federal rescheduling, and vice versa?
As of right now, state medical and recreational legalization have no impact on federal drug control laws. Although many states have chosen to legalize medical marijuana and four have legalized recreational use, those actions still violate federal law. The Obama administration has chosen to prioritize other aspects of enforcement, and has essentially turned a blind eye to these states’ experiments, so long as the state systems meet basic guidelines.
There are other avenues Congress can take besides rescheduling marijuana, however, to ameliorate the seeming breakdown in federalism brought about by marijuana policy. Congress could pass a law that would legitimate marijuana activities, but only to the extent an individual or commercial enterprise acts within the letter of state law. This would essentially function as the codification of Deputy Attorney General James M. Cole’s memoranda regarding marijuana enforcement (the “Cole Memos”). A few versions of this concept were introduced in the 113th Congress, including a bill introduced by Rep. Dana Rohrabacher [R-CA] (H.R. 1523), and another by Rep. Diana DeGette [D-CO] (H.R. 964). These adjustments and a variety of others, spanning topics from firearm sales to banking access, all died in committee. None of these is seen as a cure for the dissonance between state and federal law—nor, to be clear, do these proposals consider US obligations to international agreements—but instead, they are congressional efforts to deal with a clear policy problem.
About two dozen states have legalized medical marijuana in some form. How can there be no current accepted medical use?
State policies “affirming” the medical value of marijuana have no bearing on the determination of the FDA and DEA. In 2006, the FDA reaffirmed that they find “no sound scientific studies supported medical use of marijuana for treatment in the United States,” in response to a 2002 petition to reclassify marijuana as Schedule II. In July of 2011, the DEA formally denied this petition, repeating that marijuana has no accepted medical use and would therefore remain in schedule I. In 2013, the U.S. Court of Appeals for DC upheld that determination.
The catch-22 in the rescheduling debate is that keeping marijuana as a schedule I drug severely restricts the capacity for scientists to study its potential medical benefits, while the lack of scientific research on medical use is simultaneously offered as evidence for keeping marijuana in schedule I. If nothing else, the Surgeon General’s statements open up the possibility that the administration might make real moves for medical testing in this area—which in and of itself is a big step for marijuana advocates.(It’s worth noting that Marinol ® (dronabinol) is an FDA-approved synthetic cannabinoid that was placed in Schedule III under the CSA in 1999.)
Finally, it is a bit unclear what rescheduling of marijuana would mean for its production and sale and its “prescription” process in the states. Rescheduling of marijuana could actually complicate how states allow the prescription and delivery of the product, rather than, as advocates would prefer, liberate the process. If cannabis came under the control of FDA as a prescription drug (by whatever process), it would also be subject to tremendous testing and myriad regulatory requirements that are far beyond what states currently implement. That outcome may not be what legalization advocates would want in the end. (For more on other misunderstandings, complications and consequences of the rescheduling process and its relationship to pharmacology, read Kevin Sabet’s recent article on the topic.)
What about international law?
Proponents of keeping marijuana within Schedule I have often cited international drug conventions as an obstacle. But moving marijuana from Schedule I to Schedule II (the latter being only slightly less restrictive, as described above) would arguably still fall within the realm of possible action under international law. Three international drug conventions to which the US is a signatory allow for the controlled medical use of cannabis. Therefore, federal-level cannabis rescheduling, accomplished for strictly medical purposes and accompanied by sufficient restrictions, might be accomplished in a fashion consistent with US treaty obligations. Although, The International Narcotics Control Board—the body charged with monitoring the conventions’ implementation by member states—has examined state-level medical marijuana regimes, and found their control measures lacking.
The picture is vastly more complex for recreational cannabis activities—like those contemplated by new state legal regimes for the drug’s cultivation, purchase, sale and consumption within regulated markets. The problem has many dimensions, but the most vexing is likely this: Convention signatories are required to enact and enforce criminal laws against the production, purchase, sale and possession of “drugs,” a category to which cannabis currently belongs under the conventions’ separate scheduling regime.
Until the United Nations Commission on Narcotic Drugs (CND), which itself cannot proceed without first receiving recommendation from the World Health Organization (WHO), takes some action on cannabis, the United States will face an obligation to maintain and enforce federal criminal sanctions against most forms of recreational cannabis use, regardless of marijuana’s scheduling. Indeed, the Controlled Substances Act obligates the Attorney General to set controls on cannabis consistent with the requirements of the conventions.
The politics of rescheduling
While in an ideal world the FDA, DEA, and Attorney General would make their determinations about marijuana scheduling solely based on scientific, medical, and policy considerations, the reality is quite different. These choices are ultimately made by presidential appointees and others sensitive to political considerations. The politics surrounding administrative rescheduling are unfavorable. It is not a policy priority of the Obama administration. By all appearances, the administration believes the status quo under the Cole Memo is working. While pressure may eventually mount from a medical marijuana-friendly Surgeon General, the likely incoming Attorney General seems to have a less friendly perspective on the issue than either her predecessor or Dr. Murthy.
In addition, inaction—as well as rhetoric—from Congress suggests there is insufficient support for legislative rescheduling for marijuana. Some in Congress have been quite vocal about their opposition to administrative rescheduling. The interested actors at DOJ and HHS—as well as the White House—may be unwilling to spend political capital on this issue.
As Congress considers defunding portions of the Department of Homeland Security over the president’s immigration actions, legislators are signaling to agencies across the federal government that there can be serious repercussions for agencies that unilaterally implement policies counter to congressional interests. When Congress is willing to shut down the Department of Homeland Security over such a fight, we should expect them to be just as willing to do the same to FDA or others, and that may tell us much of what we know about the likelihood of marijuana rescheduling.
The Brookings Institution is committed to quality, independence, and impact.
We are supported by a diverse array of funders. In line with our values and policies, each Brookings publication represents the sole views of its author(s).




Commentary
How to reschedule marijuana, and why it’s unlikely anytime soon
February 13, 2015