The post here is a slightly edited version from the original, which appeared on
U.S. News and World Report
There is optimism that Congress will soon pass the 21st Century Cures bill. The draft bill proposed by the House Energy and Commerce Committee aims to foster medical innovation by streamlining the FDA regulatory process and increasing NIH research funding by $10 billion. The draft bill has overwhelming bipartisan support and will benefit patients, medical researchers, and pharmaceutical companies. However, it also includes a passage, which aims to amend the Sunshine Act and exempt pharmaceutical companies from reporting the payments they make to physicians for continued medical education (CME) programs. The supporters of this change argue that physicians learn about the latest developments in medical science through CME programs and that requiring the disclosure of these payments would discourage pharmaceutical companies from financially supporting educational programs. Ultimately they believe it could inhibit the diffusion of medical innovation among doctors.
I took a look at the data released by CMS on the financial transactions between the pharmaceutical companies and individual physicians. In the last five months of 2013, more than $120 million was paid to physicians who participated (as faculty or speakers) in CME programs. The payments constitute 26 percent of the total financial transactions between pharma and individual physicians. The proposed change essentially allows pharmaceutical companies to hide more than a quarter of their payments to physicians. Exempting the pharmaceutical companies from reporting the largest part of their financial relationship with doctors will not help to foster medical education, rather it will add to current suspicions about the unjustified impact of such payments on the drugs that physicians prescribe to their patients.
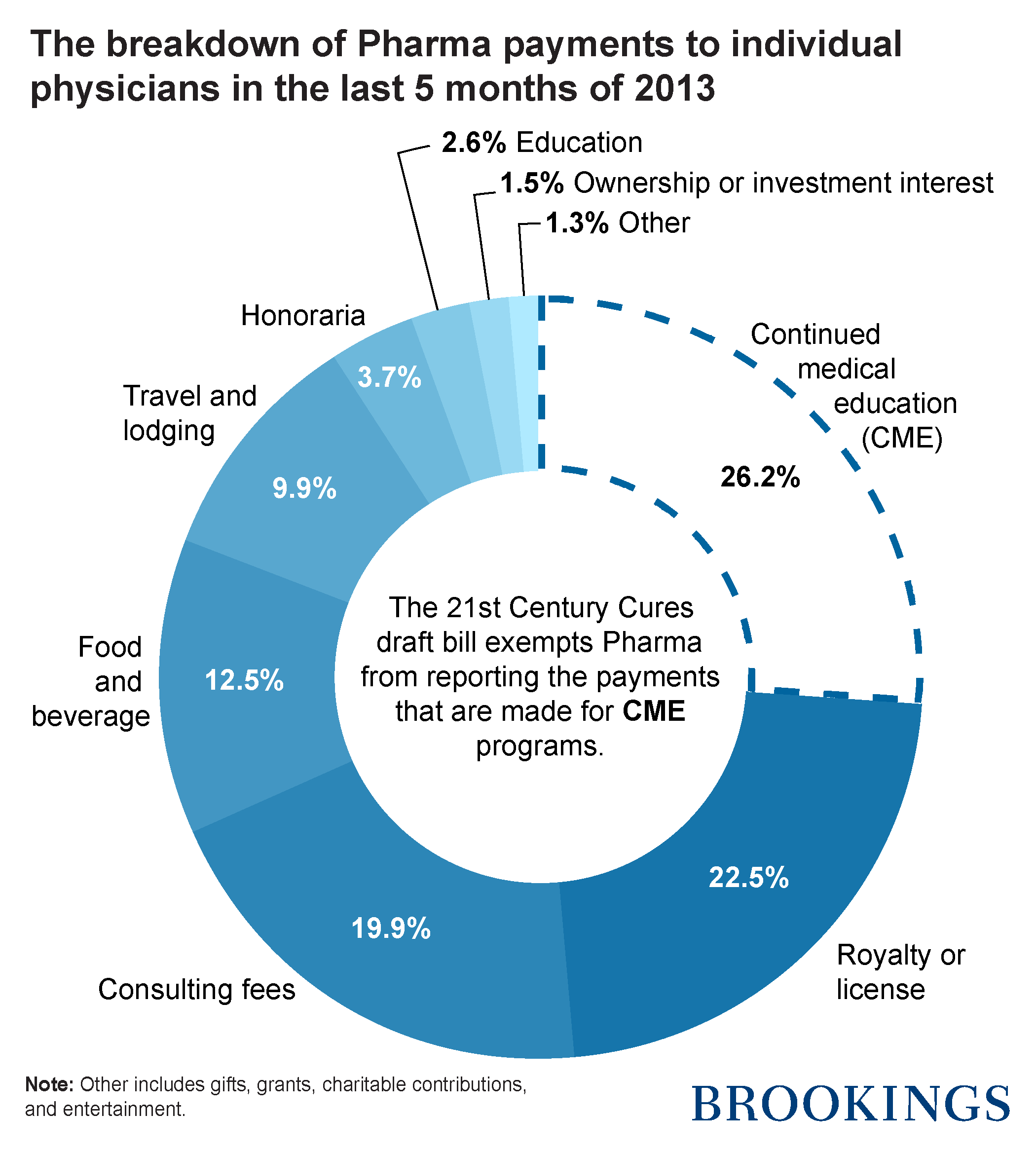
Source: CMS Data
If CME programs legitimately increase the awareness of physicians about the latest medical innovations and provide them with unbiased information about new drugs, then both pharmaceutical companies and those physicians who serve as speakers and faculties of such programs should be extremely proud of their role as champions of innovation and envoys of the latest knowledge in the medical community. If that is the case, one would wonder why they wouldn’t embrace and support the efforts that shed light on their noble role.
Patients heavily rely on the recommendations of their doctors to make any kind of decision regarding their health and thus have the right to be informed about the possibility that their doctors have a conflict of interest. Congress should refrain from amending the Sunshine Act and avoid jeopardizing the patients’ right to have access to information.
Note: I realized that in the analysis of CMS Open Payments dataset, I have incorrectly added payments made for “Compensation for services other than consulting, including serving as faculty or as a speaker at a venue other than a continuing education program” to the payments made for “Compensation for serving as faculty or as a speaker for a non-accredited and noncertified continuing education program” and “Compensation for serving as faculty or as a speaker for an accredited or certified continuing education program“. After removing the first group, CME payments would constitute 2.08 percent of the total financial transactions between pharmaceutical companies and individual physicians. This was an honest mistake and I apologize for making it. Regardless of the volume of CME payments, I still believe that Congress should refrain from amending the Sunshine act and avoid jeopardizing patients’ right to access this information


Commentary
21st Century Cures draft bill: A step backwards from transparency
May 18, 2015