Antimicrobial resistance is one of the most significant threats to public health globally. It will worsen in the coming decades without concerted efforts to spur the development of new antibiotics, while ensuring the appropriate use of existing antibiotics. Antimicrobial therapy is essential for treating and preventing bacterial infections, some of which can be life-threatening and acquired as a result of
critical medical interventions, including surgery, chemotherapy and dialysis. However, the international rise in antimicrobial resistance has weakened our antibiotic armamentarium and multi-resistant bacteria now cause over 150,000 deaths annually in hospitals around the world (WHO, 2013). Unfortunately, the evolution of drug-resistant pathogens is unavoidable due to random genetic changes in the pathogens that can render antibiotics ineffective. While antibiotic therapy can succeed in killing susceptible pathogens, it also inadvertently selects for organisms that are resistant. Because each exposure to antibiotics contributes to this process, efforts to restrict antibiotic usage only slow the development of resistance. Ultimately, innovative antimicrobial drugs with diverse mechanisms of action will be needed to treat emerging resistant pathogens.
Combating resistance
Inappropriate use of antibiotics contributes significantly to the acceleration of resistance. Needlessly exposing patients to antibiotics (for example, for viral or mild infections likely to resolve on their own), the use of overly broad-spectrum antibiotics and suboptimal doses of appropriate therapy hasten the evolution of resistant pathogens. While affordable, rapid and accurate point-of-care diagnostics are essential for determining appropriate therapy for many bacterial diseases, routine clinical use will be limited if the tests are too expensive or not accessible during routine clinical encounters. In the absence of a clear diagnostic result, many health care providers prescribe empiric broadspectrum therapy without knowing exactly what they are treating. Although inappropriate use is widespread in many parts of the world, where antibiotics are available without a prescription or oversight by a health care provider or stewardship team, overuse abounds even where antibiotic prescribing is more tightly regulated.
Studies conducted in the USA indicate that around 258 million courses of antibiotics are dispensed annually for outpatient use (Hicks, 2013) and up to 75 per cent of ambulatory antibiotic prescriptions are for the treatment of common respiratory infections, which may or may not be bacterial in origin (McCaig,1995). Recent evidence suggests that over half of these prescriptions are not medically indicated. For example, 60 per cent of US adults with a sore throat receive an antibiotic prescription after visiting a primary care practice or emergency department, despite the fact that only ten per cent require treatment with antibiotics. This is particularly troubling given the availability of rapid tests that can detect Group A Streptococcus, the bacteria responsible for the ten per cent of cases that require antibiotic treatment.
The overuse of antibiotics has been driven largely by their low cost and clinical effectiveness, which has led many patients to view them as cure-alls with few risks. This perception is reinforced by the fact that antibiotics are curative in nature and used for short durations. However, the clinical effectiveness of these drugs decreases over time, as resistance naturally increases, and this process is accelerated with inappropriate use. Moreover, there are numerous consequences associated with the use of antibiotics, including over 140,000 emergency department visits yearly in the USA for adverse incidents (mostly allergic reactions; CDC, 2013a). In addition, antibiotics can eliminate protective bacteria in the gut,
leaving patients vulnerable to infection with Clostridium difficile, which causes diarrhoeal illness that results in 14,000 deaths every year in the USA (CDC, 2013b). It is estimated that antimicrobial resistance costs the US health care system over US$20 billion annually in excess care and an additional $35 billion in lost productivity (Roberts et al., 2009).
The inappropriate use of antimicrobial drugs is particularly concerning because highly resistant pathogens can easily cross national borders and rapidly spread around the globe. In recent years, strains of highly drug-resistant tuberculosis, carbapenem-resistant Enterobacteriaceae and other resistant pathogens have spread outside their countries of origin within several years of their detection. Because resistant bacteria are unlikely to stay isolated, stewardship efforts must be improved globally and international attention is needed to improve surveillance of emerging pathogens and resistance patterns.
A major challenge for clinicians and regulators will be to find stewardship interventions that can be scaled-up and involve multiple stakeholders, including providers, drug manufacturers, health care purchasers (insurers), governments and patients themselves. Such interventions should include practical and costeffective educational programmes targeted towards providers and patients that shift expectations for antibiotic prescriptions to a mutual understanding of the benefits and risks of these drugs.
Educational programmes alone, however, will not be sufficient to lower prescribing rates to recommended levels. Pushing down the inappropriate use of antibiotics also warrants stronger mechanisms that leverage the critical relationships between the stakeholders. For example, health care purchasers can play an important role by using financial disincentives to align prescribing habits with clinical guidelines that are developed by infectious disease specialists in the private and public sectors. This type of approach has the potential to be effective because it includes multiple stakeholders that share responsibility for the appropriate use of antibiotics and, ultimately, patient care.
Key obstacles to antibiotic development
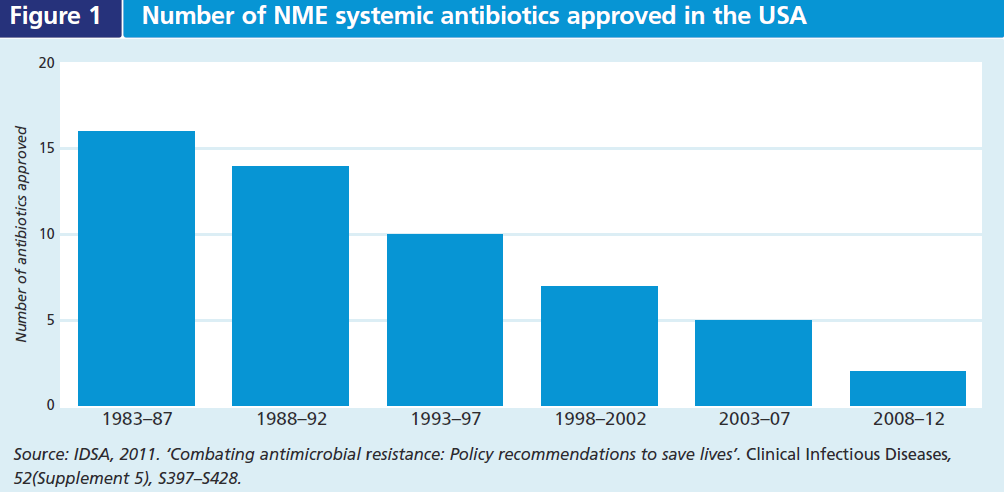
The continual natural selection for resistant pathogens despite efforts to limit antibiotic use underscores the need for new antibiotics with novel mechanisms of action. To date, antimicrobial drug innovation and development have not kept pace with resistance. The number of approved new molecular entities (NME) to treat systemic infections has been steadily declining for decades (see Figure 1). Some infections are not susceptible to any antibiotic and in some cases the only effective drugs may cause serious side effects, or be contra-indicated due to a patient’s allergies or comorbidities (e.g. renal failure). There is significant unmet medical need for therapies that treat serious and life-threatening bacterial diseases caused by resistant pathogens, as well as some less serious infections where there are few treatment alternatives available (e.g. gonorrhoea).
Antibiotic development for these areas of unmet medical need has been sidelined by a number of scientific, regulatory and economic obstacles. While the costs and complexity of any clinical trial necessary for approval by drug regulators can be substantial, in part due to the large study samples needed to demonstrate safety and efficacy, the infectious disease space faces a number of unique clinical challenges. Patients with serious drug-resistant infections may be in need of urgent antibiotic therapy, which can preclude efficient consent and timely trial enrolment procedures; prior therapy can also confound treatment effects if the patient is later enrolled in a trial for an experimental drug. In addition, many patients with these pathogens are likely to have a history of longterm exposure to the health care setting and may have significant comorbidities that render them less likely to meet inclusion criteria for clinical trials.
Emerging infections for which there are few or no treatment options also tend to be relatively rare. This makes it difficult to conduct adequate and well-controlled trials, which typically enrol large numbers of patients. However, clinical drug development can take many years and waiting until such infections are more common is not feasible. Another issue is that it may also not be possible to conclusively identify the pathogen and its susceptibility at the point of enrolment due to the lack of rapid diagnostic technologies. Ultimately, uncertainty about the aetiology of an infection may necessitate trials with larger numbers of patients in order to achieve sufficient statistical power, further compounding the challenge of enrolling seriously ill infectious disease patients in the first place.
The need to conduct large trials involving acutely ill patients that are difficult to identify can make antibiotic development prohibitively expensive for drug developers, especially given that antibiotics are relatively inexpensive and offer limited opportunities to generate returns. Unlike treatments for chronic diseases, antibiotic therapy tends to last no longer than a few weeks, and these drugs lose efficacy over time as resistance develops, leading to diminishing returns. The decline in antimicrobial drug innovation is largely due to these economic obstacles, which have led developers to seek more durable and profitable markets (e.g. cancer or chronic disease) in recent decades. There are only a handful of companies currently in the market and the development pipeline is very thin. Changes to research infrastructure, drug reimbursement and regulation are all potentially needed to revitalise antibiotic innovation.
Opportunities to streamline innovative antibiotic development
In the USA, several proposals have been made to expedite the development and regulatory review of antibiotics while ensuring that safety and efficacy requirements are met. In 2012, the US President’s Council of Advisors on Science and Technology recommended that the US Food and Drug Administration (FDA) create a ‘special medical use’ (SMU) designation for the review of drugs for subpopulations of patients with unmet medical need. Drug sponsors would be required to demonstrate that clinical trials in a larger patient population would need much more time to complete or not be feasible. A drug approved under the SMU designation could be studied in subgroups of patients that are critically ill, as opposed to the broader population, under the condition that the drug’s indication would be limited to the narrow study population. The SMU designation was discussed at an expert workshop convened by the Brookings Institution in August 2013. Many participants at the meeting agreed that there is a pressing need to develop novel antibiotics and that such a limited-use pathway could support the appropriate use of newly approved drugs.
The Infectious Diseases Society of America developed a related drug development pathway called the Limited Population Antibacterial Drug (LPAD) approval mechanism. The LPAD approach calls for smaller, faster and less costly clinical trials to study antibiotics that treat resistant bacteria that cause serious infections. Both the SMU and LPAD approaches would allow drug developers to demonstrate product safety and efficacy in smaller patient subpopulations and provide regulatory clarity about acceptable benefit–risk profiles for antibiotics that treat serious bacterial diseases. The US House of Representatives is currently considering a bill1 that incorporates these concepts.
A recent proposal from the drug manufacturer industry for streamlined antibiotic development is to establish a tiered regulatory framework to assess narrow-spectrum antibiotics (e.g. active versus a specific bacterial genus and species or a group of related bacteria) that target resistant pathogens that pose the greatest threat to public health (Rex, 2013: pp. 269–275). This is termed a ‘pathogen-focused’ approach because the level of clinical evidence required for approval would be correlated with the threat level and feasibility of studying a specific pathogen or group of pathogens. The pathogen-focused approach was also highlighted at a recent workshop at the Brookings Institution (Brookings Institution, 2014). Some experts felt that the approach is promising but emphasised that each pathogen and experimental drug is unique and that it could be challenging to place them in a particular tier of a regulatory framework. Given that pathogen-focused drugs would likely be marketed internationally, it will be important for drug sponsors to have regular interactions and multiple levels of discussion with regulators to find areas of agreement that would facilitate the approval of these drugs.
Antibiotics with very narrow indications could potentially support stewardship as well by limiting use to the most seriously ill patients. Safe use of these drugs would likely depend on diagnostics, significant provider education, labelling about the benefits and risks of the product, and the scope of clinical evidence behind its approval. Because these antibiotics would be used in a very limited manner, changes would potentially need to be made to how they are priced and reimbursed to ensure that companies are still able to generate returns on their investment. That said, a more focused drug development programme with regulatory clarity could greatly increase their odds of success and, combined with appropriate pricing and safe use provisions, could succeed in incentivising antimicrobial drug development for emerging infections.
Endnote
1 H.R. 3742 – Antibiotic Development to Advance Patient Treatment (ADAPT) Act of 2013.
References
Barnett, M. L. and Linder, J. A., 2014. ‘Antibiotic prescribing to adults with sore throat in the United States, 1997–2010’. JAMA Internal Medicine, 174(1), pp. 138–140.
Brookings Institution, 2013. Special Medical Use: Limited Use for Drugs Developed in an Expedited Manner to Meet an UnmetMedical Need. Brookings Institution. Available at:
brookings-edu-2023.go-vip.net/events/2013/08/01-special-medical-use
Brookings Institution, 2014. Modernizing Antibacterial Drug Development and Promoting Stewardship. Available at:
-development [Accessed 11 March 2014].
CDC, 2013a. Antibiotic resistance threats in the United States,2013 [PDF] CDC. Available at:
-2013/pdf/ar-threats-2013-508.pdf#page=25 [Accessed 16 January 2014].
CDC, 2013b. Clostridium difficile. Antibiotic resistance threats in the United States, 2013 [PDF] CDC. Available at:
-2013-508.pdf#page=50 [Accessed 16 January 2014].
Hicks, L. A. et al., 2013. ‘US Outpatient Antibiotic Prescribing, 2010’. New England Journal of Medicine, 368(15), pp. 1461–1463.
Infectious Disease Society of America, 2012.
Limited Population Antibacterial Drug (LPAD) Approval Mechanism. Available at:
A_News_Releases/2012/LPAD%20one%20pager.pdf [Accessed 5 March 2014].
Infectious Disease Society of America, 2012. Limited Population Antibacterial Drug (LPAD) Approval Mechanism [PDF] Infectious
Disease Society of America. Available at: A_News_Releases/2012/LPAD%20one%20pager.pdf [Accessed 18 January 2013].
Kumarasamy, K. K., Toleman, M. A., Walsh, T. R. et al.,2010. ‘Emergence of a new antibiotic resistance mechanism in India,
Pakistan, and the UK: A molecular, biological, and epidemiological study’. Lancet Infectious Diseases, 10(9), pp. 597–602.
McCaig, L. F. and Hughes, J. M., 1995. ‘Trends in antimicrobial drug prescribing among office-based physicians in the United
States’. Journal of the American Medical Association, 273(3), pp. 214–219.
President’s Council of Advisors on Science and Technology, 2012. Report to the President on Propelling Innovation in Drug
Discovery, Development and Evaluation. Available at:www.whitehouse.gov/sites/default/files/microsites/ostp/pcast-fdafinal.pdf [Accessed 5 March 2014].
Rex, J. H. et al., 2013. ‘A comprehensive regulatory framework to address the unmet need for new antibacterial treatments’. Lancet Infectious Diseases, 13(3), pp. 269–275.
Roberts, R. R., Hota, B., Ahmad, I. et al., 2009. ‘Hospital and societal costs of antimicrobial – Resistant infections in a Chicago
teaching hospital: Implications for antibiotic stewardship’. Clinical Infectious Diseases, 49(8), pp. 1175–1184.
WHO (World Health Organization), 2010. Fact Sheet: Rational Use of Medicines [webpage] WHO. Available at:
www.who.int/mediacentre/factsheets/fs338/en [Accessed 28 February 2014].
WHO (World Health Organization), 2013. Antimicrobial Drug Resistance [PDF] WHO. Available at: http://apps.who.int/gb/ebwha/pdf_files/EB134/B134_37-en.pdf [Accessed 6 March 2014].
WHO (World Health Organization), 2013. Notified MDR-TB cases (number per 100,000 population), 2005–12. WHO. Available at:
orts/G2/PROD/EXT/MDRTB_Indicators_map [Accessed 28 February 2014].



Commentary
Antimicrobial Resistance: Antibiotics Stewardship and Innovation
June 12, 2014